Description
Silver nitrate (AgNO₃) is a chemical compound that appears as a colorless to white crystalline solid. It is highly soluble in water and is commonly used in a variety of applications, both in laboratory settings and industrial processes.
Silver Nitrate for analysis, 25 gm, Merck, Germany
- Silver nitrate for analysis EMSURE® ACS, Reag, pH Eur
- Packing: 25 gm
- Brand: Merck
- Made in Germany
- Note: Here displayed product picture is not a real picture.
General Uses:
- Antiseptic: Historically, silver nitrate has been used as a topical antiseptic, especially in the treatment of burns, wounds, and infections. It’s also used to prevent neonatal conjunctivitis (eye infection) in newborns caused by Neisseria gonorrhoeae.
- Photography: Silver nitrate is a key component in the preparation of photographic materials. It reacts with light-sensitive compounds to create images on film or photographic paper.
- Chemical Synthesis: Silver nitrate is used in laboratories for various reactions, including:
- Precipitation of silver halides (e.g., silver chloride when reacted with chloride ions).
- Testing for halide ions (chloride, bromide, iodide) in solution by forming precipitates.
- Tanning Leather: Silver nitrate can be used in the tanning of leather, although it’s less common than other agents.
- Cauterizing Agent: It’s used in some medical and surgical procedures to remove warts or cauterize tissue, especially in cases where chemical cauterization is preferred over physical methods.
- Staining: It is used in microscopy and histology to stain biological tissues and make structures more visible under a microscope.
Safety and Precautions:
- Corrosive: Silver nitrate is corrosive and can cause damage to skin, eyes, and mucous membranes. Always handle it with care, wearing appropriate protective equipment like gloves, goggles, and lab coats.
- Staining: It can stain skin and clothing black or brown, so avoid contact with your skin. If silver nitrate comes in contact with the skin, it can usually be removed with a dilute solution of sodium chloride (common table salt).
- Toxicity: It can be toxic if ingested or inhaled in large quantities. Ingesting silver nitrate can lead to symptoms such as nausea, vomiting, and abdominal pain.
- Storage: Silver nitrate should be stored in airtight containers, protected from light, and kept in a cool, dry place to prevent it from decomposing.
Chemical Reactions:
- Reaction with Halides: Silver nitrate reacts with halides (chlorides, bromides, iodides) in aqueous solutions to form precipitates of silver halides, which are important in qualitative analysis:
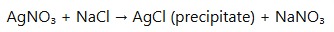
- Photographic Reaction: Silver nitrate is used in traditional photographic processes where it reacts with light to form silver metal, which creates the image on photographic film.
Silver nitrate is a versatile and important compound with many uses, especially in medicine, photography, and chemical analysis. However, it must be handled with care due to its reactive nature and potential to stain and harm skin and tissues.







Reviews
There are no reviews yet.